By Patrick Hardigan, BS, MS, PhD, Nova Southeastern University, Director of Clinical Research
Background
Fear of pain is one reason a patient may be apprehensive about a medical procedure. The most common form of pain control, namely local anesthesia, can itself produce anxiety. A variety of techniques are used to overcome this discomfort. These include alteration of factors related to the injected solution such as pH and temperature, a reduced speed of injection, and topical anesthesia.
LiDORx® is a topical anesthesia that relies on the pharmacologic effect of anesthetics when applied to surface tissue. LiDORx® releases Lidocaine Hydrochloride USP from a mild acidic vehicle to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action. A mild acidic vehicle lowers pH to increase protection against alkaline irritations and to provide a favorable environment for healing. Research demonstrates that lidocaine, used as a single agent, is effective at concentrations between 5% and 20%.
Since LiDORx® is a 3% solution we lack information regarding the onset of action. Current information suggests that the onset of action for LiDORx® is 3-5 minutes; however, this has not been verified. Given the wide variation in dose response for lidocaine, it is important for us to understand the onset of action for LiDORx®.
Study Design
Sample
Demographic Characteristics
- Gender – there was no gender-based enrollment restriction. Therefore, subjects of both genders were included in the research unless there were appropriate medical or scientific reasons. Pregnant and nursing women were not be included in the study.
- Age – there was no adult-based enrollment restriction, patients aged 18– 88 were included in the research unless there were appropriate medical or scientific reasons for their exclusion.
- Race/Ethnicity – there was no racial-based enrollment restriction, patients of all ethnicities were included in the research unless there were appropriate medical or scientific reasons.
Inclusion Criteria
- Adults between the ages of 18 and 88 years old
- Able to apply the gel
- Able to use a monofilament stick
Exclusion Criteria
- History of heart disease
- History of irregular heartbeat
- Prior MI
- An allergy to adhesives
- Allergy to lidocaine
- Taking medications, herbal remedies and/or supplements that may interact with lidocaine, including but not limited to antivirals, benzodiazepines, and St. John’s Wart
- Known liver disease
- Known kidney disease
- Adults who do not speak English
- Adults who cannot consent for him or herself
- Women who are pregnant or nursing
- Women who intend to become pregnant
Procedures
In the winter of 2014 a convenience sample of 37 subjects participated in the study. After informed consent was obtained, subjects were seated, given LiDORx® (generic packaging for blinding purposes), and a short monofilament line. A co-investigator applied the LiDORx® (according to the product directions) to the top of the subject’s non-dominant hand. Once applied, a timing device recorded the length of time to anesthesia. Time to anesthesia was articulated by the patient as yes or no through the use of the monofilament line. The subject gently poked his/her medicated hand with the monofilament line.
Outcome Measures
- Primary Outcome Measures
- Length (minutes) to onset of anesthesia
Secondary Outcome Measures
- Assess the safety and tolerability
- Evaluate local effects on the skin at the site of application for dermal adverse events
Results
Thirty-seven individuals participated in the LiDORx® study. Four patients were removed because (a) subjects never experienced the onset of anesthesia and/or (b) subjects required multiple dosages. The average age was 44.38 (± 15.90) [minimum age = 20 and maximum age = 72], 62% were female, and no adverse events were reported.
Time to Anesthesia
The median time to anesthesia was 8 minutes and 40 seconds. Over 55% of the subjects experienced anesthesia within 6 to 10 minutes.
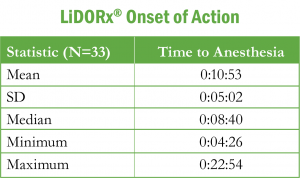
Table 1: Onset of Action
Summary
While the median time (10:53) to anesthesia exceeded the three-to-five minutes currently suggested for the 3% solution, the majority of patients experienced anesthesia within six to ten minutes. In conclusion, this study provides evidence of drug efficacy with no reported side effects.
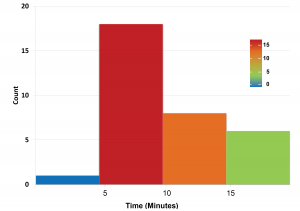
Figure 1: Onset of Anesthesia
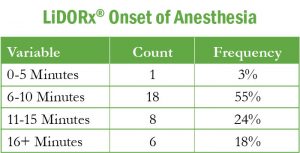
Table 2: Onset of Anesthesia
Patrick Hardigan, BS, MS, PhD, Adjunct Professor- Oceanographic Center, Professor of Public Health, Director of the Statistical Consulting Center, Nova Southeastern University
